Parmenides:
Parmenides of Elea was a pre-Socratic Greek philosopher from Elea in Magna Graecia. Parmenides has been considered the founder of ontology or metaphysics and has influenced the whole history of Western philosophy.[1]
Democritus:
In these early days most philosophers held that the earth was composed of four elements - earth, water, fire and air; but one thinker, called Democritus, a native of Thrace, explained the structure of the universe in another way. He said that it was made up of infinitely minute particles differing from each other in size, weight, shape, etc., and that these particles were indivisible, invisible, and indestructible - "atoms", he called them, i.e., particles that could not be cut in two, as the word means. At the beginning the atoms were in constant motion, but by and by they came together by chance and united in various ways to form solids, liquids and gases.[2]
Democritus, by contrast, wrote prolifically, producing over eighty known treatises, none of which have survived to the present day complete. However, a massive number of fragments and quotations of his writings have survived. These are the main source of information on his teachings about atoms. Democritus's argument for the existence of atoms hinged on the idea that it is impossible to keep dividing matter infinitely - and that matter must therefore be made up of extremely tiny particles.[3]
Democritus believed that atoms are too small for human senses to detect, that they are infinitely many, that they come in infinitely many varieties, and that they have always existed. They float in a vacuum, which Democritus called the "void", and they vary in form, order, and posture. Some atoms, he maintained, are convex, others concave, some shaped like hooks, and others like eyes. They are constantly moving and colliding into each other. Democritus wrote that atoms and void are the only things that exist and that all other things are merely said to exist by social convention. The objects humans see in everyday life are composed of many atoms united by random collisions and their forms and materials are determined by what kinds of atom make them up. Likewise, human perceptions are caused by atoms as well. Bitterness is caused by small, angular, jagged atoms passing across the tongue; whereas sweetness is caused by larger, smoother, more rounded atoms passing across the tongue.[3]
John Dalton:
The Aristotelian view of the composition of matter held sway for over two thousand years, until English schoolteacher John Dalton helped to revolutionize chemistry with his hypothesis that the behavior of matter could be explained using an atomic theory. First published in 1807, many of Dalton's hypotheses about the microscopic features of matter are still valid in modern atomic theory. Here are the postulates of Dalton's atomic theory:[4]
- Matter is composed of exceedingly small particles called atoms. An atom is the smallest unit of an element that can participate in a chemical change.
- An element consists of only one type of atom, which has a mass that is characteristic of the element and is the same for all atoms of that element. A macroscopic sample of an element contains an incredibly large number of atoms, all of which have identical chemical properties.
- Atoms of one element differ in properties from atoms of all other elements.
- A compound consists of atoms of two or more elements combined in a small, whole-number ratio. In a given compound, the numbers of atoms of each of its elements are always present in the same ratio.
- Atoms are neither created nor destroyed during a chemical change, but are instead rearranged to yield substances that are different from those present before the change.
Dalton knew of the experiments of French chemist Joseph Proust, who demonstrated that all samples of a pure compound contain the same elements in the same proportion by mass. This statement is known as the law of definite proportions or the law of constant composition.[4]
Dalton also used data from Proust, as well as results from his own experiments, to formulate another interesting law. The law of multiple proportions states that when two elements react to form more than one compound, a fixed mass of one element will react with masses of the other element in a ratio of small, whole numbers. For example, copper and chlorine can form a green, crystalline solid with a mass ratio of 0.558 g chlorine to 1 g copper, as well as a brown crystalline solid with a mass ratio of 1.116 g chlorine to 1 g copper. These ratios by themselves may not seem particularly interesting or informative; however, if we take a ratio of these ratios, we obtain a useful and possibly surprising result: a small, whole-number ratio.[4]
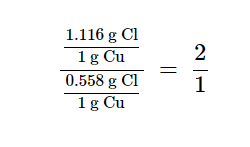
This 2-to-1 ratio means that the brown compound has twice the amount of chlorine per amount of copper as the green compound. This can be explained by atomic theory if the copper-to-chlorine ratio in the brown compound is 1 copper atom to 2 chlorine atoms, and the ratio in the green compound is 1 copper atom to 1 chlorine atom.[4]
J. J. Thomson:
A number of scientists investigated the electrical discharges that could be produced in low-pressure gases, with the most significant discovery made by English physicist J. J. Thomson using a cathode ray tube. This apparatus consisted of a sealed glass tube from which almost all the air had been removed; the tube contained two metal electrodes. When high voltage was applied across the electrodes, a visible beam called a cathode ray appeared between them. This beam was deflected toward the positive charge and away from the negative charge, and was produced in the same way with identical properties when different metals were used for the electrodes.[4]
In similar experiments, the ray was simultaneously deflected by an applied magnetic field, and measurements of the extent of deflection and the magnetic field strength allowed Thomson to calculate the charge-to-mass ratio of the cathode ray particles. The results of these measurements indicated that these particles were much lighter than atoms.[4]
Based on his observations, here is what Thomson proposed and why: The particles are attracted by positive (+) charges and repelled by negative (-) charges, so they must be negatively charged (like charges repel and unlike charges attract); they are less massive than atoms and indistinguishable, regardless of the source material, so they must be fundamental, subatomic constituents of all atoms.[4]
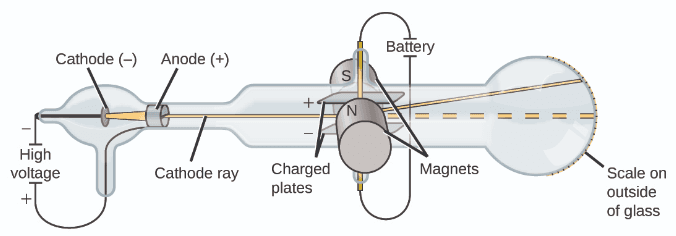
Although controversial at the time, Thomson's idea was gradually accepted, and his cathode ray particle is what we now call an electron, a negatively charged, subatomic particle with a mass more than one thousand-times less that of an atom.[4]
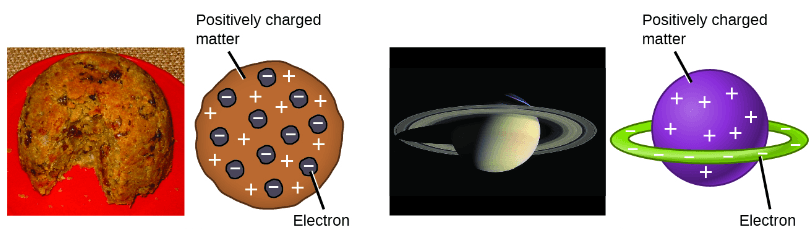
In 1904, Thomson proposed the “plum pudding” model of atoms, which described a positively charged mass with an equal amount of negative charge in the form of electrons embedded in it, since all atoms are electrically neutral. A competing model had been proposed in 1903 by Hantaro Nagaoka, who postulated a Saturn-like atom, consisting of a positively charged sphere surrounded by a halo of electrons.[4]
Robert A. Millikan:
In 1909, more information about the electron was uncovered by American physicist Robert A. Millikan via his “oil drop” experiments. Millikan created microscopic oil droplets, which could be electrically charged by friction as they formed or by using X-rays. These droplets initially fell due to gravity, but their downward progress could be slowed or even reversed by an electric field lower in the apparatus. By adjusting the electric field strength and making careful measurements and appropriate calculations, Millikan was able to determine the charge on individual drops.[4]
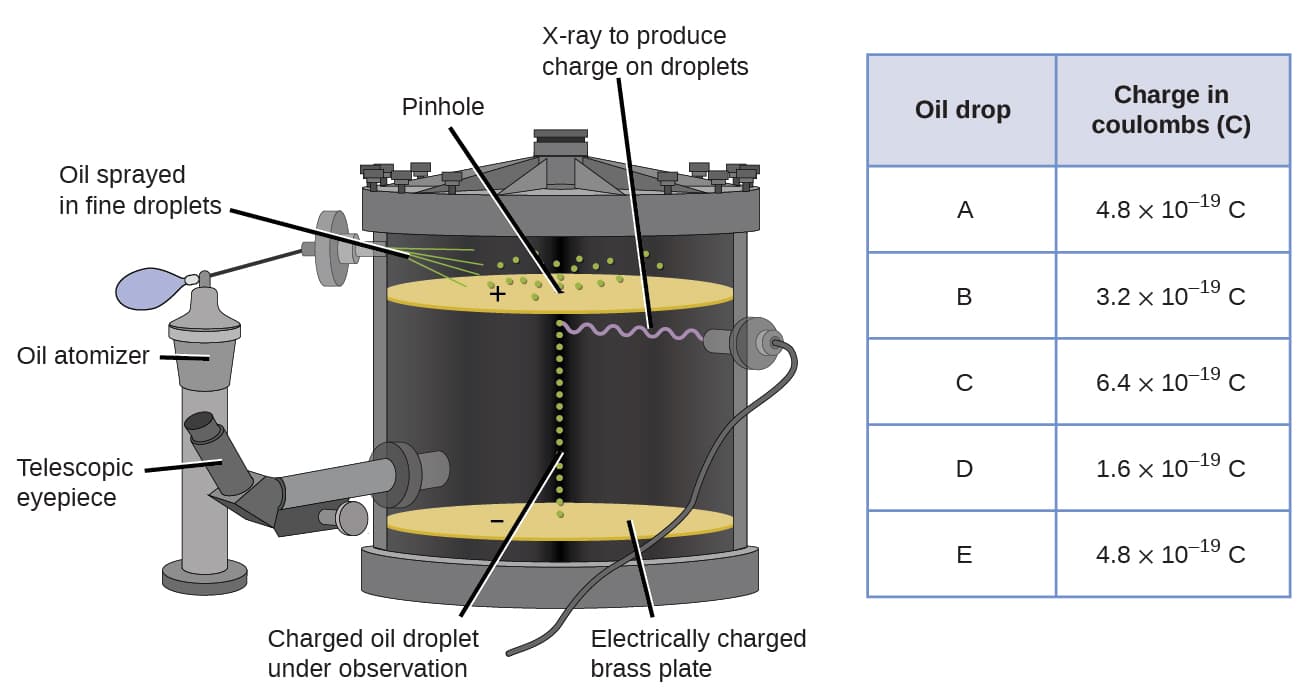
Looking at the charge data that Millikan gathered, you may have recognized that the charge of an oil droplet is always a multiple of a specific charge, \(1.6 * 10^{-19}\) C. Millikan concluded that this value must therefore be a fundamental charge — the charge of a single electron.[4]
Ernest Rutherford:
Ernest Rutherford performed a series of experiments using a beam of high-speed, positively charged alpha particles (α particles) that were produced by the radioactive decay of radium; α particles consist of two protons and two neutrons.[4]
Rutherford and his colleagues Hans Geiger and Ernest Marsden aimed a beam of α particles, the source of which was embedded in a lead block to absorb most of the radiation, at a very thin piece of gold foil and examined the resultant scattering of the α particles using a luminescent screen that glowed briefly where hit by an α particle.[4]
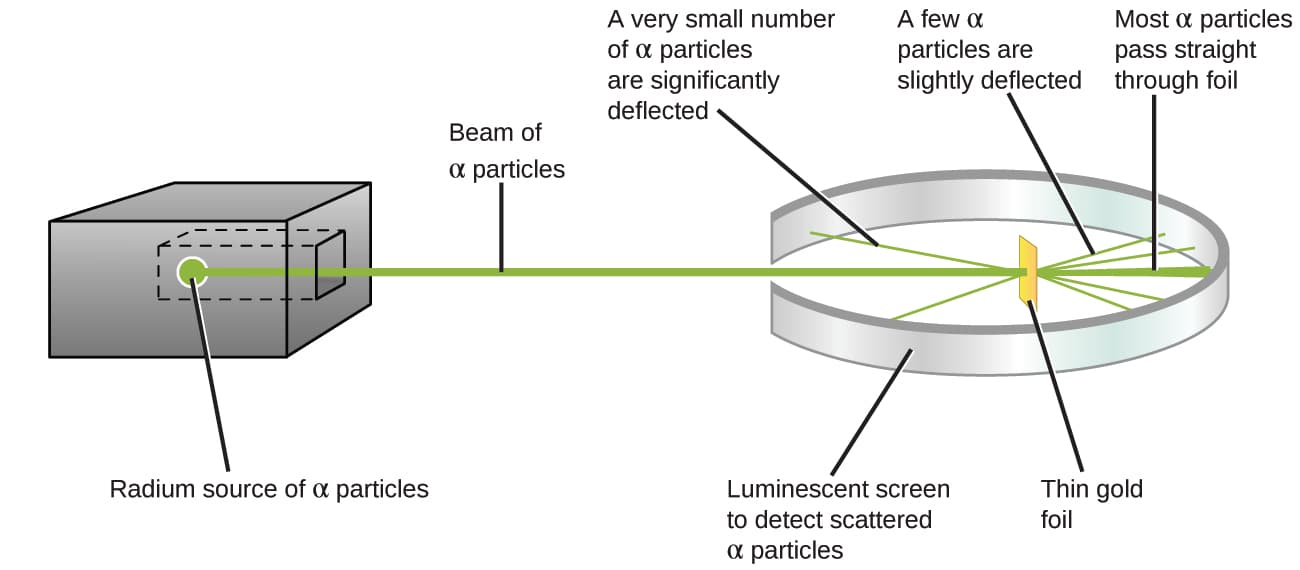
Here is what Rutherford deduced: Because most of the fast-moving α particles passed through the gold atoms undeflected, they must have traveled through essentially empty space inside the atom. Alpha particles are positively charged, so deflections arose when they encountered another positive charge (like charges repel each other). Since like charges repel one another, the few positively charged α particles that changed paths abruptly must have hit, or closely approached, another body that also had a highly concentrated, positive charge. Since the deflections occurred a small fraction of the time, this charge only occupied a small amount of the space in the gold foil. Analyzing a series of such experiments in detail, Rutherford drew two conclusions:[4]
- The volume occupied by an atom must consist of a large amount of empty space.
- A small, relatively heavy, positively charged body, the nucleus, must be at the center of each atom.
After many more experiments, Rutherford also discovered that the nuclei of other elements contain the hydrogen nucleus as a "building block", and he named this more fundamental particle the proton, the positively charged, subatomic particle found in the nucleus.[4]
Isotopes:
During the early 1900s, scientists identified several substances that appeared to be new elements, isolating them from radioactive ores. For example, a "new element" produced by the radioactive decay of thorium was initially given the name mesothorium. However, a more detailed analysis showed that mesothorium was chemically identical to radium (another decay product), despite having a different atomic mass.[4]
This result, along with similar findings for other elements, led the English chemist Frederick Soddy to realize that an element could have types of atoms with different masses that were chemically indistinguishable. These different types are called isotopes — atoms of the same element that differ in mass.[4]
Neutrons:
The nucleus was known to contain almost all of the mass of an atom, with the number of protons only providing half, or less, of that mass. Different proposals were made to explain what constituted the remaining mass, including the existence of neutral particles in the nucleus.[4]
It was not until 1932 that James Chadwick found evidence of neutrons: uncharged, subatomic particles with a mass approximately the same as that of protons. The existence of the neutron also explained isotopes: They differ in mass because they have different numbers of neutrons, but they are chemically identical because they have the same number of protons.[4]